Skip to main content
HIPEC (Hyperthermic Intraperitoneal Chemotherapy) & Cytoreductive Surgery (CRS)
Key points summarized from Dr. Healey Shulman's Grand Rounds presentation (Nov 1, 2024). Dr. Awad, Dr. Maclean & Dr. Xu have also provided insightful practical pointers.
Indications
HIPEC is indicated for the treatment of certain peritoneal surface malignancies:
-
Peritoneal carcinomatosis: secondary to cancers of the stomach, colorectal, ovary, and cervix
-
Pseudomyxoma peritonei
-
Malignant peritoneal mesothelioma
-
Sarcoma of the peritoneum.
-
Adjunct for palliative managing uncontrolled malignant ascites
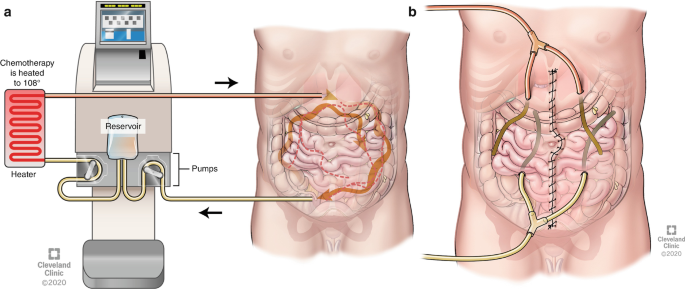
Historically, peritoneal malignancies were considered incurable and only suitable for palliation. The goal of HIPEC combined with CRS is curative for certain peritoneal surface malignancies. CRS is to surgically remove all visible tumor mass, while HIPEC aims to eradicate any remaining microscopic disease.
The heated chemotherapy solution (usually between 40°C - 43°C) is intended to increase the penetration of the chemotherapy drugs into tumor tissue. Delivering chemotherapy directly to the peritoneal cavity allows for higher local concentrations of the drug, while limiting its systemic absorption / toxicity. Heat also enhances the cytotoxicity of the chemotherapy.
Nonetheless, CRS and HIPEC still represent a radical treatment modality with high perioperative morbidity and mortality. Appropriate patient selection is key to a successful outcome. Patients should have a good baseline health / functional status / nutritional status without significant cardiac or respiratory disease and ideally be under 70 years of age. The tumor should be confined to the abdominal cavity and the patient should have no disease progression while on chemotherapy.
Surgical team also looks at tumor characteristics (load, organs involved, histopathology) during patients selection.
At Sunnybrook, HIPEC is done in OR 5 for Gynecologic Oncology service.
Anesthetic Managements
Overarching goals are to maintain hemodynamic stability, optimize organ perfusion, manage temperature fluctuations, and provide adequate analgesia, while also considering the potential side effects of chemotherapeutic agents.
Preoperative Assessment and Optimization:
-
Baseline cardiac, respiratory, and renal function, as well as nutritional status
-
Patients with ascites / pleural effusions may have compromised respiratory function, requiring assessment and management
-
Nutritional status should be evaluated, including albumin levels and any signs of sarcopenia
-
Preoperative labs CBC, extended electrolytes, renal function, LFTs, coagulation profile, G&S
-
Minimize nephrotoxins preoperatively, including NSAIDs and certain antibiotics
Intraoperative Management:
-
Hemodynamic Monitoring and Fluid Management
-
Large bore IVs & arterial line, central venous access in selected cases. Reserve one IV line for sodium thiosulphate infusion (see below). Set up the AlarisTM IV infusion pump and line for such infusion
-
The initial CRS phase involves significant fluid loss due to evaporative losses from exposed tissue and ascitic fluid drainage & potential significant blood loss
-
The HIPEC phase can cause further fluid shifts due to peritoneal inflammation
-
Goal directed fluid therapy to maintain euvolemia, optimize cardiac output and organ perfusion
-
Hemodynamics during HIPEC Phase (lasting 90 minutes):
-
Hyperdynamic State: hypermetabolic state due to the increased body temperature from the heated chemotherapeutic solution leading to an increase in heart rate, central venous pressure (CVP), and cardiac index (CI)
-
Vasodilation and Decreased Systemic Vascular Resistance (SVR): hyperthermia during HIPEC causes peripheral vasodilation, leading to a decrease in SVR and mean arterial pressure (MAP)
-
Increased Cardiac Output: The increase in heart rate is a compensatory response to the decreased SVR, aimed at maintaining cardiac output
-
Reduced Venous Return: The increased intra-abdominal pressure from the chemotherapeutic solution and the surgical procedure can decrease venous return, which may further exacerbate hemodynamic instability. The filling of the abdomen with saline and chemotherapy during the closed technique of HIPEC has a similar effect to a pneumoperitoneum, raising intra-abdominal pressure. At Sunnybrook, we adopt the closed abdomen technique. The abdomen is closed in a water-tight fashion, and the heated chemotherapy solution is circulated through inflow and outflow catheters
-
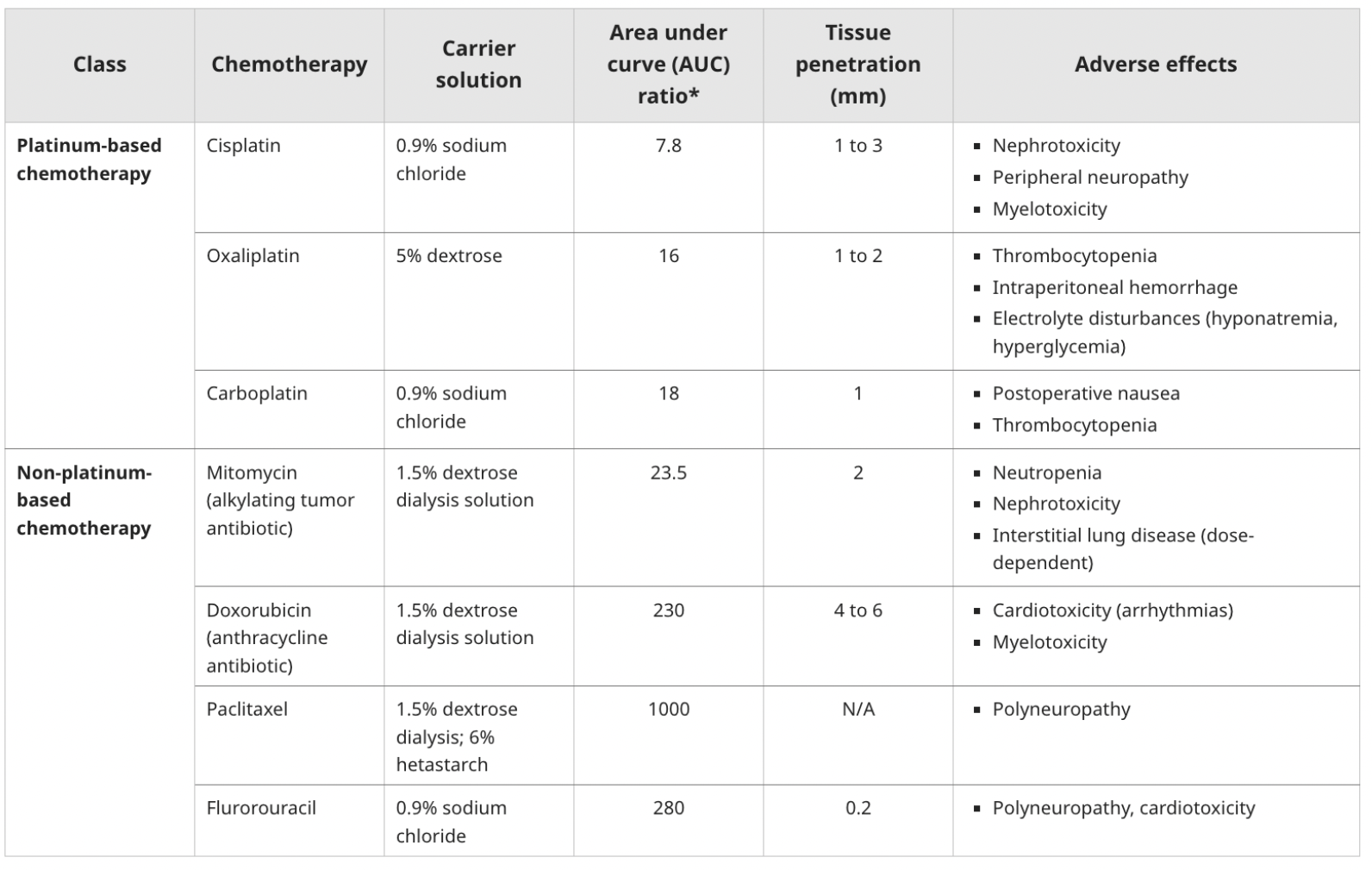
Potential for Myocardial Dysfunction The use of certain chemotherapeutic agents during HIPEC, such as intraperitoneal cisplatin, may cause cardiac toxicity and arrhythmias
-
Vasopressor may be necessary to maintain adequate MAP, particularly during the vasodilatory phase of HIPEC
-
Patients with poor cardiac reserve may require inotropic support to improve cardiac output and maintain tissue perfusion
-
Urine output particularly important during the HIPEC phase due to the potential nephrotoxic effects of chemotherapeutic drugs and hyperthermia
-
During cytoreduction (CRS): A minimum urine output of 0.5 mL/kg/hr
-
During HIPEC: The target urine output increases to 2-4 mL/kg/hr to ensure adequate renal perfusion and to help flush out nephrotoxic agents. Some sources recommend an even higher rate of at least 100 mL every 15 minutes
-
Sodium thiosulphate IV as nephroprotective agent, especially when cisplatin is used as a chemotherapeutic drug. Loading dose given15 minutes before the start of HIPEC and continues as an infusion for 6 hours after the start of HIPEC. Note that pharmacy will provide the pre-diluted sodium thiosulphate.
-
Post-HIPEC: The target urine output is reduced to 1-2 mL/kg/hr to maintain adequate perfusion and prevent fluid overload
-
Use of diuretics should only be considered once euvolemia and adequate renal perfusion are established
-
Temperature Management
-
Patients are at risk of hypothermia during the CRS phase due to large surgical exposure and fluid losses. Thus, active warming measures i.e. forced-air warmers and fluid warmer
-
During the HIPEC phase, patients are at risk of hyperthermia (increases the body's metabolic rate, leading to increased oxygen demand, heart rate, and end-tidal CO2 levels, metabolic acidosis) due to the heated chemotherapy solution. Nasopharyngeal temperature probe and additional probes in the abdominal cavity, in the inlet and outlet drains of the HIPEC machine are used
-
The core body temperature during HIPEC is to be kept below 39°C. Some sources specify a range of 37–38.8 °C as an appropriate target. Turn forced air blanket to cool, turn off fluid warmer. Ice packs around head and neck 30 mins prior to HIPEC. Lower OR room temperature. Chilled normal saline are available in the medication fridge if required (ensure bags of NS are put in the fridge the day before and are available). Place wet sheets over the patient head and arms
-
Intraperitoneal Temperature: The temperature of the chemotherapeutic perfusate within the abdominal cavity should be maintained between 41–43°C to optimize the cytotoxic effect of the chemotherapy
-
The heat exchanger on the HIPEC machine maintains the infusate temperature at 44–46°C at the machine level to ensure the intraperitoneal temperature remains within the 41–43°C range
-
-
-
The use of dextrose-containing carrier solutions for chemotherapeutic agents can cause significant hyperglycemia, especially with oxaliplatin
-
Hyponatremia can occur due to the diffusion of sodium into the peritoneal solution and intravascular fluid shifts caused by hyperglycemia. This is more common with dextrose-containing carrier solutions.
-
Hypokalemia
-
Hypocalcemia
-
Chemotherapeutic agents like cisplatin can cause hypomagnesemia
-
Cisplatin and oxaliplatin, have direct toxic effects on renal function which further exacerbate electrolyte and acid-base disturbances
-
Frequent ABG e.g. q15-30min is recommended during HIPEC
-
Coagulopathy
-
Major fluid shifts, blood loss, transfusion causing dilutional coagulopathy
-
Analgesia:
These procedures typically require large midline abdominal incisions and extensive tissue manipulation.
-
Thoracic epidural - be mindful about possible pre-existing coagulopathy caused by preoperative chemotherapy and nutritional deficiencies
-
Multimodal analgesia including the use of intravenous opioids, regional blocks (rectus sheath, ESP, TAP), and non-opioid analgesics, such as acetaminophen, Ketamine / dexmedetomidine / lidocaine infusion. Caution with NSAIDs in the context of nephrotoxicity
Anti-emetic:
High risk for PONV secondary to GA, chemotherapy and female gender.
-
Ondansetron 8mg & Dexamethasone 10mg IV prior to HIPEC
-
Standing order of Dexamethasone & Ondansetron IV post op for 48-72 hours (ordered by the surgical team)
-
For refractory case, Aprepitant (Emend) (non-formulary) can be obtained through pharmacy. Consult pharmacy for proper dosing
Chemotherapy Considerations:
The chemotherapeutic agents used in HIPEC can have various systemic side effects, including nephrotoxicity, cardiotoxicity, myelosuppression, and electrolyte imbalances.
-
PPE - chemo protective gowns (will be provided in OR), chemo safe gloves or double gloves. Powder-free gloves are recommended as powder is known to absorb cytotoxic agents and increase exposure. Some centres recommend shoe covers and eye wear for droplet protection. Our nursing team has been trained with the safe handling / disposal of chemo, and will be responsible for running the chemo pump
Post op disposition: PACU then HISSUU


